Annotations:
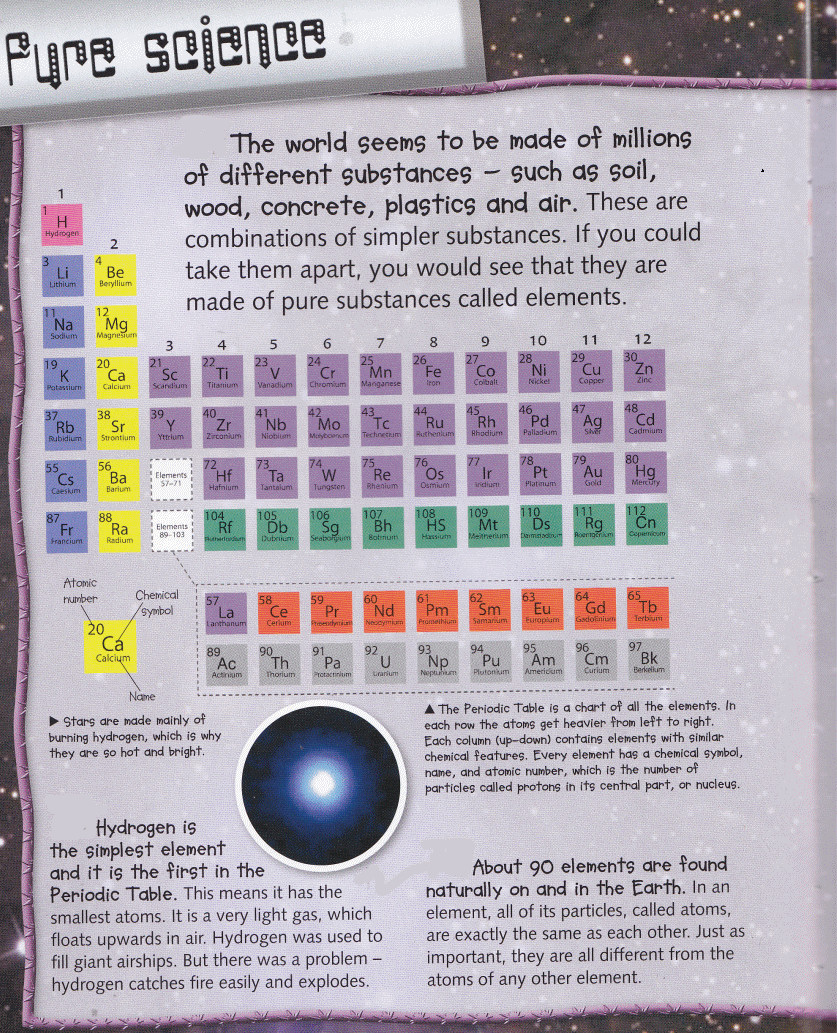
alkali metal (Alkalimetall) - The alkali metals are a group (column) in the periodic table consisting of the chemical elements lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr). The alkali metals have very similar properties: they are all shiny, soft, highly reactive metals at standard temperature and pressure. They can all be cut easily with a knife due to their softness, exposing a shiny surface that tarnishes rapidly in air due to oxidation.
alkaline earth metals - are metallic elements found in the second group of the periodic table (e.g. magnesium, calcium, strontium). All alkaline earth elements have an oxidation number of +2, making them very reactive. Because of their reactivity, the alkaline metals are not found free in nature.
transition metals - the 38 elements in groups 3 through 12 of the periodic table are called "transition metals" (e.g. titanium, vanadium, cadmium). They all conduct electricity and heat. The interesting thing about transition metals is that their electrons they use to combine with other elements, are present in more than one shell. Iron, cobalt, and nickel are the only elements known to produce a magnetic field.
halogens - the name 'halogen' means 'salt-producing'. When halogens react with metals they produce a wide range of salts, including calcium fluoride, sodium chloride (common salt), silver bromide and potassium iodide.
inert gas ( Edelgas) - An inert gas is a gas which does not undergo chemical reactions under a set of given conditions. The noble gases often do not react with many substances. Inert gases are used generally to avoid unwanted chemical reactions (such as oxydation or hydrolysis).
lanthanides - the lanthanide series of chemical elements comprises the fifteen metallic chemical elements with atomic numbers 57 through 71, from lanthanum through lutetium. These fifteen lanthanide elements, along with the chemically similar elements scandium and yttrium, are often collectively known as the rare earth elements.
actinides - the actinide series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. All actinides are radioactive and release energy upon radioactive decay; naturally occurring uranium and thorium, and synthetically produced plutonium are the most abundant actinides on Earth. These are used in nuclear reactors and nuclear weapons. Uranium and thorium also have diverse current or historical uses, and americium is used in the ionization chambers of most modern smoke detectors.
Assignments:
1. Answer the 4 Quiz questions.
2. What are the particles called in the nucleus of an atom?
3. What element gives off rays and dangerous particles?
4. Why is uranium harmful as well as beneficial?
5. Which element does not rust and is extremely light?
|